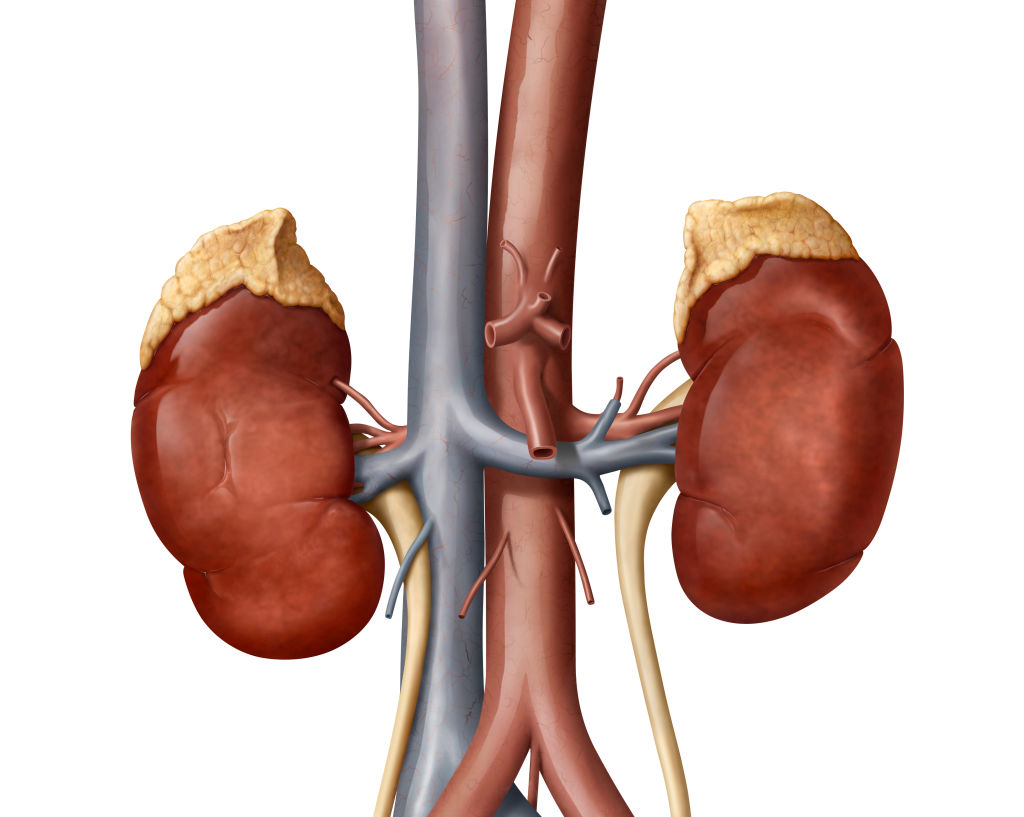
The FDA has approved Neurocrine Biosciences’ Crenessity, a new therapy for rare endocrine disorder Congenital Adrenal Hyperplasia (CAH). Designed to block the corticotropin-releasing factor type 1 receptor and thus inhibit secretion of ACTH, the drug reduces the production of adrenal androgens. Crenessity, which comes in a twice-daily pill formula for adults and an oral solution for children aged four and older, is the first new CAH treatment in 70 years.

NSA warns that overlooked botnet technique threatens national security
The National Security Agency (NSA) has warned that fast flux, a technique used by cybercriminals and hostile nations to hide their activities, is a significant