Company appoints leading sales, market access and operations executives with decades of experience successfully launching and commercializing new oncology products
New Drug Application (NDA) for avasopasem is currently under priority review by the U.S. Food and Drug Administration (FDA) with a…
Source: www.marketscreener.com – Read more
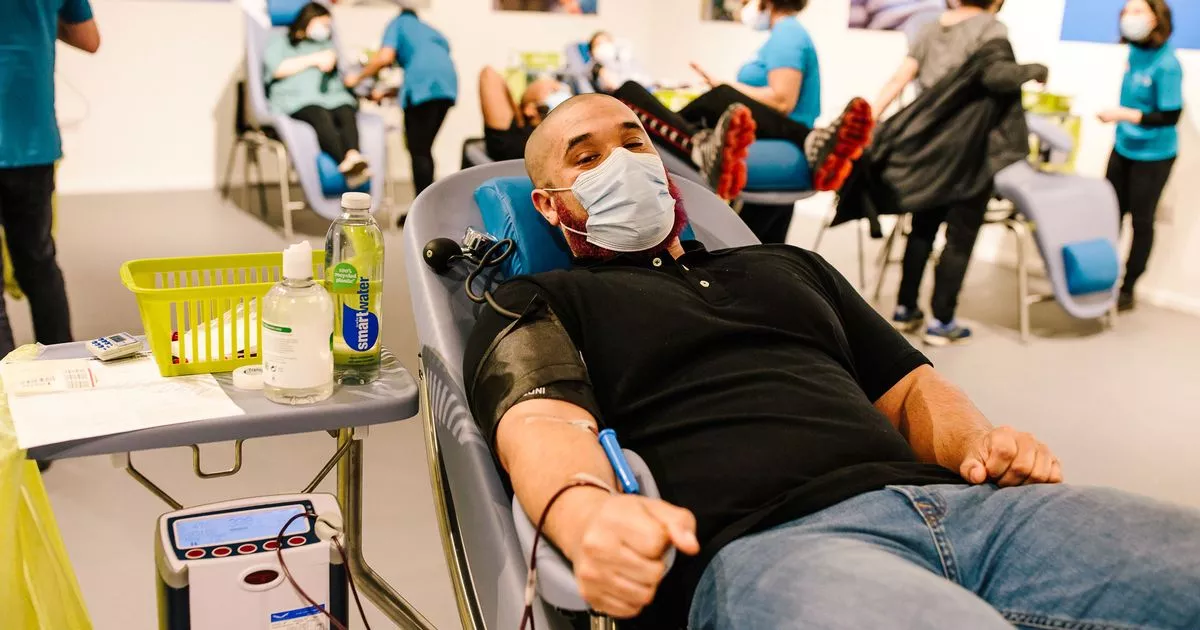
‘Amber alert’ as NHS in Plymouth makes urgent plea for people with certain blood type
The NHS has issued an urgent call for O type blood donors, following increased demand after the recent cyber attack. The attack led to reduced



