The FDA has had a busy December 2025, with several notable new drug approvals. Eli Lilly’s Zepbound became the first FDA-approved medicine for chronic sleep apnea. Meanwhile, drugs for rare diseases were also given the go-ahead, including a treatment by Ionis Pharmaceuticals for a rare metabolic disorder and Humacyte’s bioengineered blood vessel for trauma patients. Finally, the FDA approval was granted to the first cell therapy from mesenchymal stromal cells by Mesoblast for graft versus host disease.
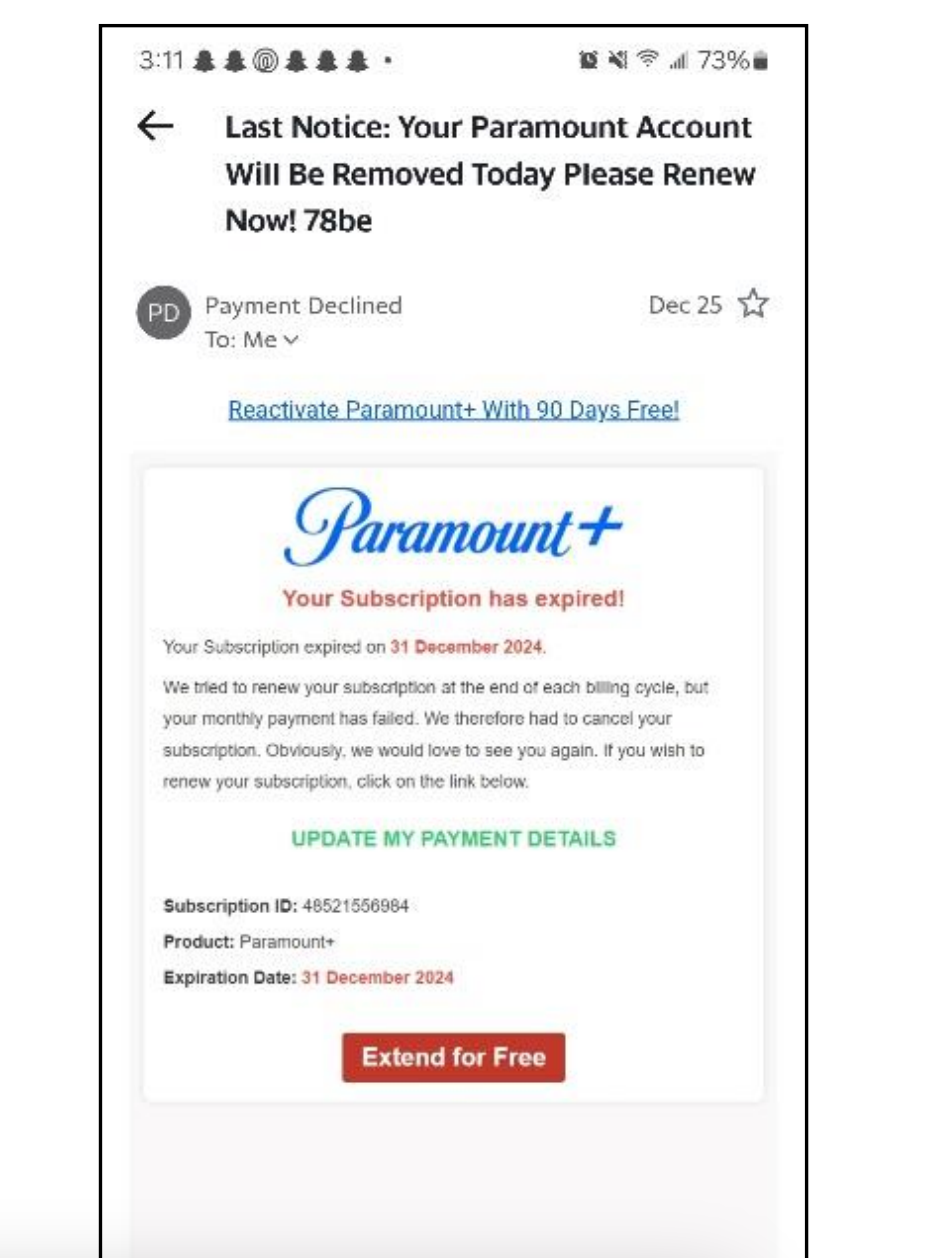
New email scam in Kan. could place malware on your computer
Sedgwick County District Attorney, Marc Bennett, warns residents about new email scams that could infect their computers with malware. He advises against clicking links that


