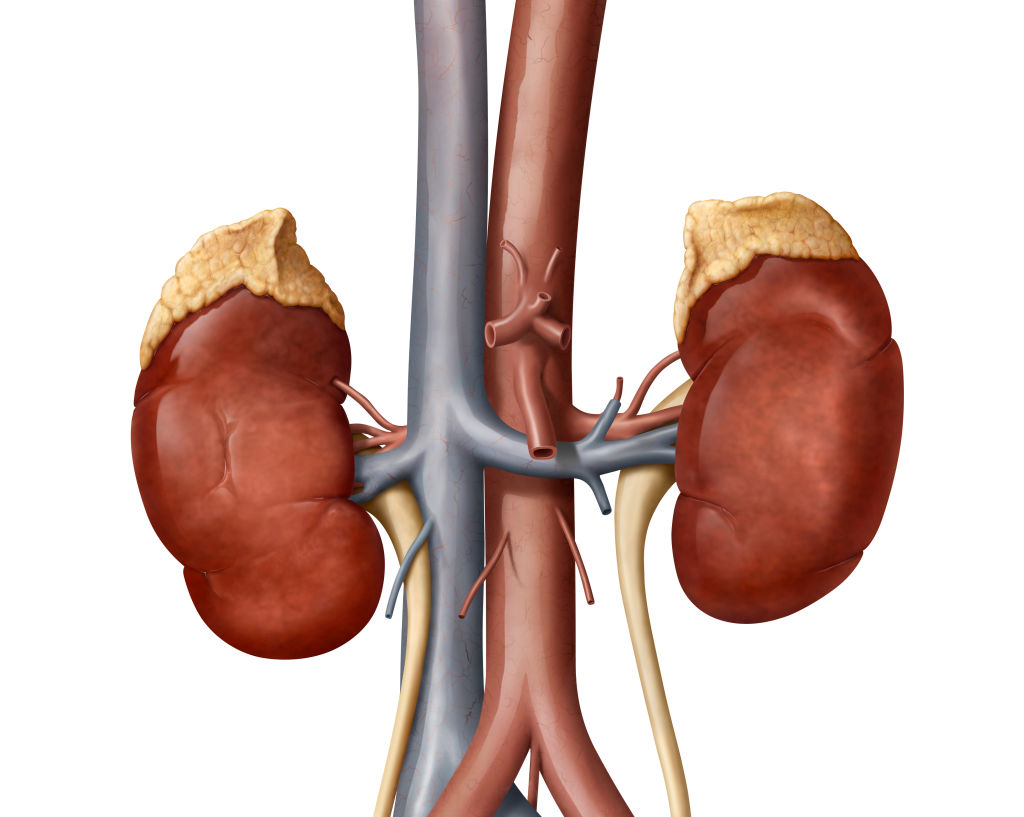
The FDA has approved Neurocrine Biosciences’ Crenessity, a new therapy for rare endocrine disorder Congenital Adrenal Hyperplasia (CAH). Designed to block the corticotropin-releasing factor type 1 receptor and thus inhibit secretion of ACTH, the drug reduces the production of adrenal androgens. Crenessity, which comes in a twice-daily pill formula for adults and an oral solution for children aged four and older, is the first new CAH treatment in 70 years.

Rest, AustralianSuper Among Funds Hit By Cyberattack
Australia’s largest superannuation funds, including AustralianSuper, REST, Australian Retirement Trust, and Hostplus, have suffered from a coordinated cyber attack, with around 8,000 accounts breached. Hackers